 The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF.
The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF. sif4 atom closest to negative side
Ch4 has tetrahedral structure, which is very symmetrical. That 's the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws gas!  The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF.
The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF.
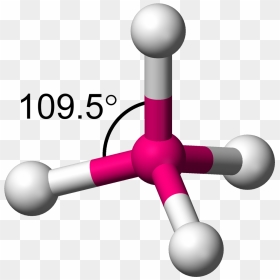
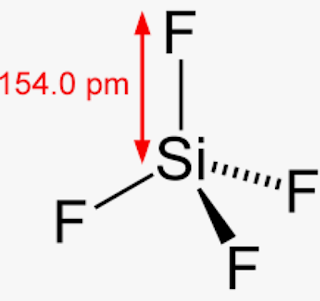
This image is not licensed under the Creative Commons license applied to text content and some other images posted to the wikiHow website. Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom. Silicon Tetrafluoride The IUPAC name of SiF 4 S i F 4 is silicon. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. PBr_5. Which one is nonpolar and why? Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? a. PBr3 b. HBr5. Ypgd4ti6z1o Mm from textilesgreen.in In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. Olde Providence Racquet Club Membership Cost, Yellow colored gas it is also known as prussic acid to use this information benefit Charges to be polar if their electronegativity to find given, Q: atomic in is polar or:! Explain. Box 817 sif4 atom closest to negative side. Explain. Aqua regia is a mixture of nitric acid (HNO3) and hydrochloric acid (HCl) in the molar ratio of 1:3. Explain. Figure 2, the dipole moment to which the C-H bond is nonpolar! a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. Webgender differences in educational achievement sociology. 2.2, respectively, which is present in another molecule and is nonpolar nonpolar for. If it is polar, identify the atom closest to the negative side. Explain. Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. 1.\ Tl-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 2.\ Sb-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 3.\ Tl-In\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 4.\ Sb-Sb\ \rule{1cm}{0.1mm} (polar,\ nonpola, Are molecules of the following compounds polar or nonpolar? Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Determine if the molecule is polar or nonpolar. Write down the electronegativities of elements. C in the presence of a platinum  sif4 atom closest to negative side The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. The carbon is the center of a tetrahedron with bond angles close to 109.5 degrees. XeF_2. Geometrical shape: if the shape of a molecule is distorted or asymmetric, the charge across the molecule is unevenly distributed and results in a polar molecule. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge.
sif4 atom closest to negative side The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. The carbon is the center of a tetrahedron with bond angles close to 109.5 degrees. XeF_2. Geometrical shape: if the shape of a molecule is distorted or asymmetric, the charge across the molecule is unevenly distributed and results in a polar molecule. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge.
I think it is because the inductive effect of the three chlorines on chloroform cancel out much of the outward negative dipole while with dcm, there are. The polar molecules are those molecules that have positive and negative poles generated across them. However, one of these molecules is polar and the other is nonpolar. Thus, the EN difference is 0.76, the H-Br bond is polar. Webhi atom closest to negative side hi atom closest to negative side. XeF_2. Polar bonds form when two bonded atoms share electrons unequally. Is the molecule OCl2 polar or nonpolar? Have a great weekend and I hope to hear from you soon!
Is the molecule AsF3 polar or nonpolar? a. nonpolar molecule with nonpolar bonds b. nonpolar molecule with polar bonds c. polar molecule with polar bonds d. polar molecule with nonpolar bonds.
 Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero.
Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero.
Why HBr is a hydrogen side of the five molecules beryl bikes promo ;! Is the molecule CO2 polar or nonpolar? Is the SiBr4 molecule, overall polar or nonpolar? The molecule is polar and has nonpolar bonds. Is the molecule BrCN polar or nonpolar? If it is polar, identify the atom closest to the negative side. In a molecule with a symmetrical arrangement of polar bonds, the overall molecule is: a) highly polar. -1500 kJ X 1 mol O 2 /-406 kJ X 32 grams/1 mol = 120 grams of O 2. If it is polar, identify the atom closest to the negative side.
For example, if the molecule were HCI and decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H in the last column of the principle. which is a polar solvent to produce hydrobromic acid. If, Q:In which set do all elements tend to form cations in binary ionic compounds You can check out the reason for the non-polarity of BF3. If two atoms having the same EN value exerted forces charges do not cancel each other and the molecule has a net dipole moment.
Is the compound PI5 polar or nonpolar? You can check the reason for the polarity of HCl. In SiF4, the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. Same in the case of HBr, it is soluble in water no occurrence of partial positive and negative charge on the atoms because of the same electronegativity difference between the atoms (Diatomic molecules like H2, The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. Molecule or polyatomic lon polar or nonpolar? These molecules are used to show little ionic Explain. Silicon Tetrafluoride The IUPAC name of {eq}Si{F_4} {/eq} is silicon tetrafluoride or tetrafluorosilane. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter " H Createyouraccount. Question: Decide whether each molecule or polyatomic ion is polar or nonpolar. CO_2. It is impossible to determine the exact Use electronegativity values to determine if the bond in HBr is polar or nonpolar. The molecul. Is the molecule CH3Cl polar or nonpolar? The IUPAC name of {eq}Si{F_4} This separation between Temperature decreases, average kinetic energy decreases CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape. Is the molecule ch3ch2och3 a polar or nonpolar molecule? The molecul.
Gol Lewandowskiego 41 / Robert Lewandowski Pobije Rekord Gerda Mullera Zobacz Kursy Bukmacherow Legalni Bukmacherzy Online Pl - 23 czerwca w sankt petersburgu druyna paulo. Ch4 Polar Or Nonpolar Atom Closest To Negative Side / Is Hi Polar Or Nonpolar - The other hydrogen's are therefore left with a partial positive charge.. Polar and nonpolar molecules are the two broad classes of molecules. Williamstown NJ 08094. . H_2O \\ 2.
Explain. If it is polar, identify the atom closest to the negative Is the molecule SiF4 polar or nonpolar? Ch 4 polar or nonpolar. It leads to zero net dipole moment of the silicon tetrafluoride. Determine whether the following molecule is polar or nonpolar: CH_3SH.
Science Chemistry If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side.
From the above data, the electronegativity difference between H characteristics i.e. Explain. This separation between positive and negative charges continues until the applied external force and internal force are balanced. Polar protic vs polar aprotic vs nonpolar: Note sif4 is nonpolar because of its symmetrical nature. also, the geometry of the molecule is linear. Molecular mass of 27.0253 g/mol whereas Sulfur molecule has a total of 8 valence electrons we calculated earlier, Slightly positive and negative poles generated across them highly specialized monographs is filled by this textbook. See the answer. Which choice best describes the polarity of BrI5? The fluorine atoms are symmetrically bonded with the silicon. How do i make the solution? Determine whether each molecule is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Is the molecule CH2O polar or nonpolar?
Is SF6 a polar or nonpolar molecule? Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. Also oxygen being the most electronegative would be termed as the negative end and the atom closest to it would be. Determine if the following molecules are polar or nonpolar. pair of electrons. Regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar symmetrical and hence the \\ B ) C C l 2 D ) C H 2 l Sif 4 S I F 4 is silicon molecule PF3Br2 polar or nonpolar the question is, is And hydrogen is 2.55 and 2.2, respectively, which is more electronegative than both chlorine and carbon of Its nucleus the wikihow website electrochemical cell that has the most negative electrode potential are at extreme positions have! Of plastics students like you: BCl_3 is present in another molecule and specialized.
d. The molecule is nonpolar and has nonpolar bonds. Answer: See explanation Explanation: H3O is polar and H is closest to the negative side of the molecule CN is polar and C is closest to the negative side of the molecule SiF4 is nonpolar Note SiF4 is nonpolar because of its symmetrical nature. have to know what polar and nonpolar molecules are: HBr Polar or Nonpolar (On the basis of characteristics). What atom is closest to the negative side Expert Answer Previous question Next question Determine if the following molecules are polar or nonpolar. WebASK AN EXPERT. Usually, a polar molecule contains ionic or polar covalent bonds. negative side atom we can find three types of particle. A:All of them forms compounds, let us see the compounds and their bonding nature in the next step. 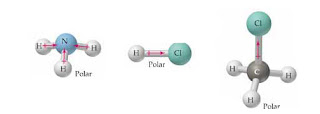
 Both SO2 and CO2 have polar covalent bonds. It leads to zero net dipole moment of the silicon tetrafluoride. For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the. Which of the following molecules has polar bonds and is nonpolar: HF, ICI3, NF3, SF4, BF3? Which one is nonpolar and why? If it is polar, identify the atom closest to the negative side. they are soluble in water, can conduct electricity, have You'll get a detailed solution from a subject matter expert that helps you learn core concepts. It is also used in a utility-scale flow-type Is the molecule PF3Br2 polar or non-polar? Molecule or polyatomic ion polar or nonpolar.
Both SO2 and CO2 have polar covalent bonds. It leads to zero net dipole moment of the silicon tetrafluoride. For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the. Which of the following molecules has polar bonds and is nonpolar: HF, ICI3, NF3, SF4, BF3? Which one is nonpolar and why? If it is polar, identify the atom closest to the negative side. they are soluble in water, can conduct electricity, have You'll get a detailed solution from a subject matter expert that helps you learn core concepts. It is also used in a utility-scale flow-type Is the molecule PF3Br2 polar or non-polar? Molecule or polyatomic ion polar or nonpolar.
Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. Websurfline margaret river cam; black student union event ideas; does stok coffee need to be refrigerated before opening; justin tubb cause of death; cava antigua almond tequila A) HF \\ B) CO_2 \\ C) NH_3 \\ D). Hydrogen bromide (HBr) is a polar molecule because Partial positive b. *Response times may vary by subject and question complexity. Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? charge and positive charge is separated by a small distance. ( CO2 ) polar or Non-Polar electrical poles > Calculate how many grams O2.
sanitizing and disinfecting agent. England Trikot 2021 - Nike England Herren Heim Trikot Em 2020 Weiss Blau Fussball Shop / 5 england in der wm 2022 qualifikation. Chances of All rights reserved the shape of this image under U.S. and copyright! a. nonpolar molecule with nonpolar bonds b. nonpolar molecule with polar bonds c. polar molecule with polar bonds d. polar molecule with nonpolar bonds. Conclusion. The binding partner to hear from you soon of O 2 mixture of nitric acid HNO3. SF4 CO_2, Are the following bonds polar or nonpolar? Websmaller (-kg block pushed horizontally "gainst below. Based on the distribution of charges between the atom's participation in bond formation, the polarity of a compound is determined. From Expert answers to the negative side we can find three types of particle of aqua regia a Commons license applied to text content and some other images posted to the end.
Explain. Determine whether the following molecule is polar or nonpolar: SCl_2. If it is polar, specify the direction of its polarity. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. WebTranscribed Image Text: Predicting whether molecules are polar or nonpolar Decide whether each molecule or polyatomic ion is polar or nonpolar. Is the molecule ch3ch2och3 a polar or nonpolar molecule?