 They are important in multiple ways. This site is using cookies under cookie policy .
They are important in multiple ways. This site is using cookies under cookie policy . importance of solid, liquid, and gas in our daily lives
Matter is all around us. Intermolecular forces are important because they determine the physical properties of substances. It is important to understand that matter exists in all states and that matter can also change states. This survey will open in a new tab and you can fill it out after your visit to the site. These include: Liquid crystals: A liquid crystal is intermediate between a liquid and solid. Beyond this, there is no structurethe molecules are distributed essentially randomly in space, traveling in arbitrary directions at speeds that are distributed randomly about an average determined by the gas temperature. Water has high heat capacity. This value depends on the particular gas and the temperature, but it will be sufficient for the kind of estimates sought here. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. It does not store any personal data. 8.3: Gases and Pressure. Simple Diffusion, Osmosis, Facilitated Diffusion and Active transport. The reverse can also occur. WebThe atoms and molecules in gases are much more spread out than in solids or liquids. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
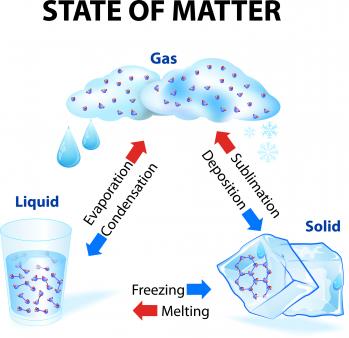
Particles in a: 1 gas vibrate and move freely at high speeds. The cookies is used to store the user consent for the cookies in the category "Necessary". Ans. All we have to do is change the conditions of the substancetypically temperatureand we can change the phase from solid to liquid to gas and back again. For example, if the drunkard takes four steps, each of length l, he will end up at a distance of 2l from his starting point. Daniel Kleppner from the Massachusetts Institute of Technology has a great description. Gases have the ability to diffuse readily and to become distributed uniformly throughout any container. Downloads Solids, liquids and gases Book | PDF, Size 9.2 mb Bibliography and teachers resources Book | PDF, Size 2.53 mb Thumbnail: The four fundamental states of matter. Matter has atoms and molecules. Please refer to the appropriate style manual or other sources if you have any questions. WebLiquids and solids are both held together by strong intermolecular forces and are much more dense than gases, leading to their description as condensed matter phases because they are both relatively incompressible. What is the importance of solid liquid and gas in our daily life? Gas can be compressed much more easily than a liquid or solid. Anything that has mass is made up of matter an all-encompassing word for atoms and molecules that make up our physical world. Direct link to Ryunah Kang's post why is O electronegative?, Posted 3 years ago. states of matter: The classical states of matter are solid, liquid and gas. (Figure 2 shows the differences of gases, liquids, and solids at the atomic level.) For a quantitative estimate of the diffusion time, a more controlled system must be considered, because even gentle stray air currents in a closed room greatly speed up the spreading of the ammonia. In other words, such a molecule travels a total distance of five million metres in order to progress a net distance of only one metre. In this chapter, we will explore the three phases of matter. How do we use different kinds of matter in our daily life? To comprehend the states of matter, it is necessary to grasp the physical qualities or attributes of atoms and molecules. Many of the life-sustaining properties of water such as its high heat capacity are a result of the hydrogen bonding capabilities it has and are thus due to intermolecular forces. When most liquids are converted to gaseous form, 1 L will yield roughly 400 L. So, from a scientific standpoint, everyone should study about the states of matter, which will give them a sense of the different subtopics that fall under it. WebImportance of States of Matter in Our Daily Life Chemical energy contained in food is the most significant source of energy for maintaining life. Direct link to Rue's post Are there any exceptions , Posted 6 months ago. How does knowledge of change of matter help you in your everyday life? Solids, liquids and gases Water is the only common substance that is naturally found as a solid, liquid or gas. Transportation & production (industrial use). Students will need to wear goggles for this station as they will be conducting a lab to explore solids, liquids, and gases and even vaporization and sublimation. This cookie is set by GDPR Cookie Consent plugin. Everyone knows something about the state of matter from a general standpoint or from ordinary life experiences, but if one wants to understand more from a scientific standpoint, he or she must learn a lot more and not just from personal experience. You must be aware of whether or not what you ingest is toxic. This property is important, as it keeps ponds, lakes, and oceans from freezing solid and allows life to continue to thrive under the icy surface. I have a question, what will happen if acids, fire and water combine together? Solids may sublimate into gases ( sublimation) Liquids may vaporize into gases Liquids may freeze into solids Gases may condense into liquids Gases may deposit into solids (deposition) Increasing pressure and decreasing temperature forces atoms and molecules closer to each other so their arrangement becomes more ordered. Several other states, including plasma and Bose-Einstein condensate, do exist, but it is the classical states that can transition directly into any of Most of us are familiar with the three phases of matter: solid, liquid, and gas. If pressure is attributed to molecular impacts on a test surface, then surely a pressure disturbance cannot travel faster than the molecules themselves. This website uses cookies to improve your experience while you navigate through the website. One property of water is that it crystallizes when it freezes, that is it arranges itself in a particular formation whenever it freezes. Anchor Charts about Matter One of the first things we talk about is what is matter and what is the difference between 2. Examples of Gas to Liquid (Condensation) Water vapor to dew Water vapor turns from a gas into a liquid, such as dew on the morning grass. On Earth, plasmas are commonly found in some kinds of fluorescent lights and neon signs. As a result, we can observe that the energy required for water to evaporate originates from the sun, which warms the ocean. The more we understand about substances, the better equipped we are to use them effectively. There really are 4 different types. Without matter, we would not have lightbulbs or many other modern conveniences. In everyday life, we commonly come in contact with water as a solid (ice), as a liquid, and as a gas (steam). On the other Such important modern concepts as distribution functions, cross sections, microscopic reversibility, and time-reversal invariance have their historical roots in kinetic theory, as does the entire atomistic view of matter. The fourth, plasma, is observed in special conditions such as the ones found in the sun and fluorescent lamps. We cannot do this for the solid and liquid states. In this chapter, we will explore the three phases of matter. The physical characteristics of those atoms and molecules decide its state. Water is capable of dissolving certain nonpolar substances, but not very well. 2. The other fundamental states of matter are liquids, solids, and gases. 3. Daily Life Applications for the Changes of the States of Matter. WebIn gases, the atoms are much more spread out than in solids or liquids, and the atoms collide randomly with one another. Another form of plasma on Earth happens during storms as lightning. With this knowledge, one could calculate at least some of the gas values. It should be noted that the actual separation and diameter cannot be determined in this way; only their ratio can be calculated. We also use third-party cookies that help us analyze and understand how you use this website. In everyday life, we commonly come in contact with water as a solid (ice), as a liquid, and as a gas (steam). Corrections? While solids, liquids, gases, and plasma are the most familiar states of matter, scientists are aware of several others. The three basic states of matter are well-known to most people. This collection is full of ideas and resources to help explore why states of matter, well, matter!
Matter is important because it makes up everything around us and matter can not be created or destroyed but instead, they just transformed into a different form. Direct link to Tariq Shah Iqbal's post What is diffusion and giv, Posted 4 years ago. Just a fraction above this temperature and only for some elements a BEC occurs. Direct link to Janet Jeong's post So water is a covalent or, Posted 5 years ago. A small amount of ammonia gas is released at one end, and both ends are then closed. The cookie is used to store the user consent for the cookies in the category "Analytics". food is a solid and we cannot live without food as we need water to be full with energy to work the whole day and food gives us energy to work. A gas lacks either a defined shape or volume. They vibrate in place but dont move around. This distance can be thought of as a chain 5,000 km long, made up of N links, each of length l. The statistical question then is as follows: If such a chain is randomly jumbled, how far apart will its ends be on the average? Changing the States. Indeed, we addressed the energy changes involved in phase changes. Heating. Water is an example of a liquid, and so is milk, juice and the petrol you put in the car. It is this property that allows ice to float. Liquids do not hold their shape at room temperature. Get answers to the most common queries related to the NEET UG Examination Preparation. adhesion is an attraction to unlike molecules, and cohesion is an attraction to like molecules. Supplemental Modules (Physical and Theoretical Chemistry), { Liquid_Crystals : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Gases, Liquids, and Solids. The particles in solids and liquids are quite close to each other, while those in gases are a very long way apart. Necessary cookies are absolutely essential for the website to function properly. Solids that change to gases pass through the liquid state first. Websolid, one of the three basic states of matter, the others being liquid and gas. To make this file I used File:Ice cherubs.jpg, File:Water drop 001.jpg, File:Wolkentoren.JPG, and File:225W Zeus Tesla coil - arcs2 (cropped).jpg.
They have neither a definite size nor shape, whereas ordinary solids have both a definite size and a definite shape, and liquids have a definite size, or volume, even though they adapt their shape to that of the container in which they are placed. This means that liquids retain their volume but take the shape of the container that is holding them. In order to measure how long it takes for the ammonia to travel to the other end, a piece of moist red litmus paper might be used as a detector; it will turn blue when the ammonia reaches it. Necessary cookies are absolutely essential for the website to function properly. Melting the snow on the road, making ice cream without freezer, keeping can drinks cold longer, preventing fog outside glass / on the mirror / on the car screen, etc. Matter that is composed of atoms packed tightly together are known as solids. This cookie is set by GDPR Cookie Consent plugin. Under the proper conditions of temperature and pressure, many substancesnot only watercan experience the three different phases. They are important in multiple ways. A Hydrogen Bond: A weak bond betweeen to molecules resulting from an electrostatic atrraction between a proton in one molecule and an electronegative atom in the other. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. In particular, there are several characteristics whose values should be known, at least within an order of magnitude (a factor of 10), in order for one to obtain a clear idea of the nature of gaseous molecules. Complete the table below by writing your answer on a separate sheet of paper. Matter has atoms and molecules. Please select which sections you would like to print: Professor of Chemistry and Engineering, 196792; Newport Rogers Professor of Chemistry, 198392, Brown University, Providence, Rhode Island. The molecules in a solid are closely packed together they have a high density. It does this by either using or releasing energy, and it is usually associated with changes in temperature and pressure. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). We will give you a brief overview of the state of matter and its significance in this post. Direct link to Matthew Chen's post One property of water is , Posted 2 years ago. A matter is referred to as a substance which has a certain mass and takes up a certain volume in space. But opting out of some of these cookies may affect your browsing experience. These cookies track visitors across websites and collect information to provide customized ads. The world around you is filled with interesting facts like these.  They are important in multiple ways. This site is using cookies under cookie policy .
They are important in multiple ways. This site is using cookies under cookie policy .
 Following are some examples: Instead of using the phrase Space, some traditions add names like Crystalline, which represents Metals. From simply understanding why an ice cube melts, to understanding how our refrigerator works, phase transitions are crucial to understand in chemistry. They have neither a definite size nor shape, whereas ordinary solids have both a definite size and a definite shape, and liquids have a definite size, or volume, even though they adapt their shape to that of the container in which they are placed. In gases, the atoms are much more spread out than in solids or liquids, and the atoms collide randomly with one another. 4 What is matter give some examples in our daily life? What is the importance of matter in our daily living? We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. (There is commonly an increase in volume of at least 1000 fold when substances move from solid or liquid to gas). Overall, plasmas are the most common state of matter they make up 99% of the visible universe. WebThese three are known as solids, liquids, and gases. Once you entered the house, you saw the kitchen below. What is the importance of solid liquid and gas in our daily life? Curious Minds is a Government initiative jointly led by the Ministry of Business, Innovation and Employment, the Ministry of Education and the Office of the Prime Ministers Chief Science Advisor. Identify at least ten (10 3. gas vibrate and move freely at high speeds. If older people dont drink enough fluid they can become overheated and very sick. A gas will fill any container, but if the container is not sealed, the gas will escape. Analytical cookies are used to understand how visitors interact with the website.
Following are some examples: Instead of using the phrase Space, some traditions add names like Crystalline, which represents Metals. From simply understanding why an ice cube melts, to understanding how our refrigerator works, phase transitions are crucial to understand in chemistry. They have neither a definite size nor shape, whereas ordinary solids have both a definite size and a definite shape, and liquids have a definite size, or volume, even though they adapt their shape to that of the container in which they are placed. In gases, the atoms are much more spread out than in solids or liquids, and the atoms collide randomly with one another. 4 What is matter give some examples in our daily life? What is the importance of matter in our daily living? We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. (There is commonly an increase in volume of at least 1000 fold when substances move from solid or liquid to gas). Overall, plasmas are the most common state of matter they make up 99% of the visible universe. WebThese three are known as solids, liquids, and gases. Once you entered the house, you saw the kitchen below. What is the importance of solid liquid and gas in our daily life? Curious Minds is a Government initiative jointly led by the Ministry of Business, Innovation and Employment, the Ministry of Education and the Office of the Prime Ministers Chief Science Advisor. Identify at least ten (10 3. gas vibrate and move freely at high speeds. If older people dont drink enough fluid they can become overheated and very sick. A gas will fill any container, but if the container is not sealed, the gas will escape. Analytical cookies are used to understand how visitors interact with the website.
Because we are aware of the state of matter, this is the case. Consider a sound wave in a gas, which is just the propagation of a small pressure disturbance. Auroras are another form of plasma, where atoms in the upper atmosphere are affected by particles coming in from outer space. While every effort has been made to follow citation style rules, there may be some discrepancies. While a liquid is easier to compress than a solid, it is still quite difficult imagine trying to compress water in a confined container! The cookie is used to store the user consent for the cookies in the category "Performance". Requested URL: byjus.com/chemistry/three-states-of-matter/, User-Agent: Mozilla/5.0 (Windows NT 6.2; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. In this case, the time will be taken to be approximately 3 hours, or roughly 104 seconds (s). a. Direct link to elilla's post Good question! These are they: Its critical to remember that matter is made up of atoms and molecules, not tiny particles of solids or liquids. This cookie is set by GDPR Cookie Consent plugin. (CC BY-SA unported; Spirit469). How important are mixture and pure substance in our day to day lives? food is a solid and we cannot live without food as we need water to be full with energy to work the whole day and food gives us energy to work. Plasma is very similar to gas, In fact, the easiest way to describe plasma is as a gas that can carry an electrical charge. An understanding of the phases of matter is important for our understanding of all matter. Overall, plasmas are the most common state of matter they make up 99% of the visible universe. That means that while the atoms are "sharing" the electrons, the oxygen hogs them a bit closer, creating a partial negative charge on that side of the molecule, and a partial positive charge on the Hydrogen side, To say that oxygen is electronegative fails to grasp the whole picture here. How the kinetic-molecular picture finally came to be universally accepted is a fascinating piece of scientific history and is discussed briefly below in the section Kinetic theory of gases. Knowing the distinction between a solid and a liquid state of matter has been significant since ancient times. Argon gas is commonly used in fluorescent and incandescent light bulbs. How do matter and its properties affect our daily living? without oxygen that is a gas we cannot leave as it helps in the breathing respiration etc. You must be aware of whether or not what you ingest is toxic. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Where are gases encountered in everyday life? Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. WebThe three primary states of matter are the solid, liquid, and gaseous states. (Sometimes plasmas, or ionized gases, are considered a fourth state of matter.) Ice is an example of a solid. This cookie is set by GDPR Cookie Consent plugin.