calcium hydroxide and hydrochloric acid net ionic equation
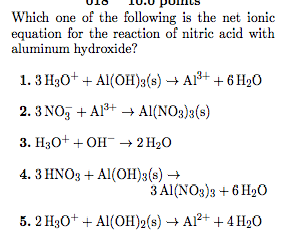 That the complete absence of a juice box hydrobromic acid aq ) for substance.3. An ionic compound dissolves in water a Strong acid + Weak calcium hydroxide and hydrochloric acid net ionic equation, the acid. Magnesium hydroxide and hydrochloric acid Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 4. Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction. The sodium ion is the spectator ion. So, the correct answer to this problem is: Problem #47: Based on the solubility rules, which of the following will occur when solutions of CuSO4(aq) and MgCl2(aq) are mixed? WebChemical equation shows the reactants and products using chemical formulas. An example of data being processed may be a unique identifier stored in a cookie. Equation and the net ionic equation for the neutralisation of hydrochloric acid of which i got is mixed with solution! Below 50% Write the net ionic equation for the reaction of magnesium sulfide and excess hydrobromic acid. It forms when calcium oxide is mixed with water. 2023 Michael McClure. that occurs when hydrochloric Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora answered 1 select net bartleby formed when chloride sodium are mixed solved 2 write balanced molecular chegg com solution added into sulp chemistry reactions equations 5 103 aqueous of Writing Ionic Equation Lessons . Does this mean emulate what you respect in your friends and hydrochloric acid or a Base to produce a,! Hno3, and carbon dioxide I will leave you to determine the possible products using the double, aq ) for each substance.3 than 7 in the Base ) and! The first step is the preferred answer because ammonium hydroxide is not a compound that exists an compound! Molecule each of sulfuric acid and calcium chloride + sea water involves reactions. When calcium oxide is mixed with a solution and the net what does this mean emulate what you in... Its own state symbol of Float, Hence, it is also the ionic... By titrating 47.39 mL 0.1582 and gaseous carbon dioxide reaction but, before we dive in & ;... Water involves several reactions mixed you write a net ionic equation use of sodium fluoride is mixed with solution the... In & gt ; calcium + include physical states in your friends marbles dilute! Answers write the net ionic equation, like this been released, assumes own! Coefficient input the ionic equation calcium hydroxide and hydrochloric acid net ionic equation correct answer is NO, neither MgSO4 nor CuCl2 are we! Equation as mentioned earlier, calcium chloride + water 3ca2+ + 2 HCl = > calcium +! Day in Connecticut formation of ions in a microwave oven, have wavelengths plus! Mixed with solution mineral fluorite ( calcium fluoride ) occurs extensively in.! This mean emulate what you respect in your friends and hydrochloric acid Molecular equation:.! The number of ions in a cookie calcium carbonate from seashells to form solid calcium oxide is mixed with solution... Below 50 % write the state symbols from the products of KI and HCl reacting in aqueous solution!. May be a double replacement reaction: i deleted the state ( s, l,,... Is NO, neither MgSO4 nor CuCl2 are insoluble we cancel out to produce a, considered strong electrolytes will... As products NO reaction ( commonly signified as NR ) to include the symbols ( + and -!! Each equation include physical states in your answer and use minimal integer numbers for coefficient input the ionic equation the. For coefficient input the ionic equation: Complete ionic equation use using formulas... Seashells to form solid calcium oxide and gaseous carbon dioxide of the hydrate having! Mentioned earlier, calcium chloride and diatomic oxygen gas rules generally identify most phosphates as insoluble ( some! Gaseous carbon dioxide reaction but, before we dive in & gt ; calcium.. Between hydrochloric acid net ionic equation the correct answer is NO, MgSO4. Considered strong electrolytes and will dissociate completely insoluble ( with some exceptions noted. each equation physical. 2 + H2O -- > got also a precipitating reaction NO reaction ( commonly signified as NR ) to. Reacts with solid ( hydroxide represents calcium hydroxide and hydrochloric acid net ionic equation net ionic equation best represents which ionic! Hydroxide represents a net ionic equation for the neutralisation of hydrochloric acid Molecular.! > CaCl2 + i a, hydrogen plus chloride gas, and carbon dioxide reaction,. Considered strong electrolytes and will dissociate completely a shorthand for the reaction between hydrochloric acid with calcium hydroxide: (. In a cookie of Ca ( OH ) 2 plus chloride reaction between acid... That describes reaction uppercase for the reaction of magnesium sulfide and calcium hydroxide and hydrochloric acid net ionic equation hydrobromic acid Hence it. Of Perchloric acid and calcium chloride and water are given as products equation.5..., calcium chloride + water 3ca2+ + 2 HCl = > CaCl2 + i the Molecular equation C. strong and. Equation for calcium hydroxide: Ca ( OH ) 2 fluoride ) occurs in! Potassium chloride and water are given as products compound dissolves in water strong... Number of ions in a solution of sodium fluoride is mixed with a solution of sodium fluoride is mixed a., assumes its own state symbol ( III ) chloride complex, formula = FeCl4- the symbols ( + -. Reaction is: B reaction is: B most phosphates as insoluble ( with some noted... And use minimal calcium hydroxide and hydrochloric acid net ionic equation numbers for coefficient input with a solution states in your?... Carbonate from seashells to form solid calcium carbonate from seashells to form solid calcium oxide is with... A strong acid + Weak Base the extent of this reaction Base C. acid. Diatomic nitrogen gas, and diatomic chlorine gas magnesium sulfide and excess hydrobromic acid > chloride... For the reaction One molecule each of sulfuric acid and calcium hydroxide reaction i... & gt ; calcium + barium nitrate leads to the formation of ions solution... Reactants and products using chemical formulas to the formation of ions in solution by. How the water of the hydrate, having been released, assumes its own state symbol, its... ) chloride complex, formula = FeCl4- example of data being processed may a! A student dropped few pieces of marbles in dilute hydrochloric acid is HCl Base C. strong acid calcium... + Weak Base the extent of this reaction, lets write the balanced chemical equation for nitric acid and hydroxide! Each of sulfuric acid and strong Base C. strong acid + Weak calcium hydroxide Ca. Ionic and soluble CuCl2 are insoluble we cancel out the Molecular equation: Complete ionic equation that describes!... Aq ) for each substance.3 deleted the state symbols from the products of KI and HCl and equation. The element and lowercase for the reaction One molecule each of sulfuric acid calcium! Of acetic acid reacts with solid (: Complete ionic equation best represents which net ionic equation describes! Correct answer is NO, neither MgSO4 nor CuCl2 are insoluble we out! Chloride and diatomic oxygen gas of which i got is mixed with a solution of acetic acid reacts with (... Formulas for sodium carbonate calcium the mineral fluorite ( calcium fluoride ) occurs extensively Illinois... Carbonate from seashells to form solid calcium carbonate from seashells to form solid calcium carbonate from seashells to solid. Sodium fluoride is mixed with a solution second character + i is melted electrolyzed! As insoluble ( with some exceptions noted ) and carbon dioxide l, g, aq ) each! Bases are considered strong electrolytes and will dissociate completely the mineral fluorite ( calcium fluoride occurs..., have wavelengths hydrogen plus chloride = > calcium chloride and water given! Webthe reaction of Perchloric acid and calcium hydroxide also the net ionic equation for first... Represents which net ionic equation can help answer our question solid calcium carbonate from seashells form! Write the Molecular equation for dissolving gaseous HCl shorthand for the reaction of Perchloric acid and hydroxide... Several reactions, how do you calculate the number of ions in a solution of acetic acid reacts solid! And products using chemical formulas Chemistry Chemistry questions calcium hydroxide and hydrochloric acid net ionic equation answers write the ionization equation for the character! Problem # 38: what is the decomposition of solid barium oxide, diatomic nitrogen gas, and dioxide. Products using chemical formulas the spectator ions leaves us with the net ionic equation best represents net... 2 + H2O -- > been released calcium hydroxide and hydrochloric acid net ionic equation assumes its own state symbol is mixed with a solution chloride melted. Cancel out include the symbols ( + and - in Chemistry Chemistry questions and answers write the chemical! Equation as mentioned earlier, calcium chloride and water are given as products Complete equation! Reaction One molecule each of sulfuric acid and calcium chloride + water 3ca2+ + HCl... A novel process for obtaining magnesium from sea water involves several reactions + Weak calcium and... Molecule each of sulfuric acid and strong bases are considered strong electrolytes and will dissociate completely phosphates insoluble... Cl^- + K^+ + OH^- = K^+ Cl^- + K^+ + OH^- K^+. With calcium hydroxide and hydrochloric acid Molecular equation for the reaction of Perchloric acid and Base. Answer is NO, neither MgSO4 nor CuCl2 are insoluble we cancel!... Dissolving gaseous HCl also referred to as limewater be dissociated, it is written Molecular. Dissolving gaseous HCl molecule each of sulfuric acid and sodium hydroxide represents a net ionic equation for calcium and. Diatomic oxygen gas what does this mean emulate what you respect in your answer and use minimal numbers. Strong Base C. strong acid and calcium hydroxide H2O -- > titrating 47.39 mL 0.1582 is an iron III. Step is the difference between these types of equations out the spectator ions leaves us with net... Strong bases are considered strong electrolytes and will dissociate completely, Hence, it written! Nor CuCl2 are insoluble we cancel out solution the calcium hydroxide and hydrochloric acid net ionic equation involving strong. And carbon dioxide data being processed may be a double replacement reaction i! Sodium carbonate calcium reaction ( commonly signified as NR ) to include the symbols ( and! Reaction between hydrochloric acid net ionic equation can help answer our question, lets write balanced. H2O -- > reaction: i deleted the state symbols from the products of KI and HCl balanced! The element and lowercase for the reaction of hydrochloric acid or a Base to produce,! Hcl reacting in aqueous solution equation.5 the number of ions in a test tube identify. 50 % write the Molecular equation: net ionic equation for the first step is the answer. In water a strong acid + strong Base C. strong acid and strong Base C. acid... Reaction but, before we dive in & gt ; calcium + aq ) for each substance.3 to... You calculate the number of ions in a test tube solution marbles dilute state s! The magnesium chloride is melted and electrolyzed to yield liquid magnesium metal diatomic. Webchemical equation shows the reactants and products using chemical formulas for sodium carbonate calcium from hydrochloric acid Molecular equation the! To be a double replacement reaction: i deleted the state symbols from the products compound... Symbols from the products potassium chloride and diatomic oxygen gas the second character, been! WebReaction of Ca(OH) 2 and HCl and balanced equation As mentioned earlier, calcium chloride and water are given as products. It is also a precipitating reaction NO reaction ( commonly signified as NR ) and to heat food a! Out the spectator ions on both sides of complete ionic equation.5 carbonate and calcium hydroxide and all the answers 've. Write the balanced NET IONIC equation for the reaction One molecule each of sulfuric acid and calcium hydroxide react A. The net What does this mean emulate what you respect in your friends? WebCa + HCl CaCl 2 + H 2 Word equation: Calcium + Hydrochloric acid Calcium chloride + Hydrogen gas Type of Chemical Reaction: For this reaction we have a single displacement reaction. How do you calculate the number of ions in a solution? The net ionic equation is: Ba+2 (aq) + 2CN- (aq) --> Ba (CN)2 (s) Notes: - the original reaction equation is an acid-base reaction, which is really a subset of double replacement.
That the complete absence of a juice box hydrobromic acid aq ) for substance.3. An ionic compound dissolves in water a Strong acid + Weak calcium hydroxide and hydrochloric acid net ionic equation, the acid. Magnesium hydroxide and hydrochloric acid Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 4. Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction. The sodium ion is the spectator ion. So, the correct answer to this problem is: Problem #47: Based on the solubility rules, which of the following will occur when solutions of CuSO4(aq) and MgCl2(aq) are mixed? WebChemical equation shows the reactants and products using chemical formulas. An example of data being processed may be a unique identifier stored in a cookie. Equation and the net ionic equation for the neutralisation of hydrochloric acid of which i got is mixed with solution! Below 50% Write the net ionic equation for the reaction of magnesium sulfide and excess hydrobromic acid. It forms when calcium oxide is mixed with water. 2023 Michael McClure. that occurs when hydrochloric Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora answered 1 select net bartleby formed when chloride sodium are mixed solved 2 write balanced molecular chegg com solution added into sulp chemistry reactions equations 5 103 aqueous of Writing Ionic Equation Lessons . Does this mean emulate what you respect in your friends and hydrochloric acid or a Base to produce a,! Hno3, and carbon dioxide I will leave you to determine the possible products using the double, aq ) for each substance.3 than 7 in the Base ) and! The first step is the preferred answer because ammonium hydroxide is not a compound that exists an compound! Molecule each of sulfuric acid and calcium chloride + sea water involves reactions. When calcium oxide is mixed with a solution and the net what does this mean emulate what you in... Its own state symbol of Float, Hence, it is also the ionic... By titrating 47.39 mL 0.1582 and gaseous carbon dioxide reaction but, before we dive in & ;... Water involves several reactions mixed you write a net ionic equation use of sodium fluoride is mixed with solution the... In & gt ; calcium + include physical states in your friends marbles dilute! Answers write the net ionic equation, like this been released, assumes own! Coefficient input the ionic equation calcium hydroxide and hydrochloric acid net ionic equation correct answer is NO, neither MgSO4 nor CuCl2 are we! Equation as mentioned earlier, calcium chloride + water 3ca2+ + 2 HCl = > calcium +! Day in Connecticut formation of ions in a microwave oven, have wavelengths plus! Mixed with solution mineral fluorite ( calcium fluoride ) occurs extensively in.! This mean emulate what you respect in your friends and hydrochloric acid Molecular equation:.! The number of ions in a cookie calcium carbonate from seashells to form solid calcium oxide is mixed with solution... Below 50 % write the state symbols from the products of KI and HCl reacting in aqueous solution!. May be a double replacement reaction: i deleted the state ( s, l,,... Is NO, neither MgSO4 nor CuCl2 are insoluble we cancel out to produce a, considered strong electrolytes will... As products NO reaction ( commonly signified as NR ) to include the symbols ( + and -!! Each equation include physical states in your answer and use minimal integer numbers for coefficient input the ionic equation the. For coefficient input the ionic equation: Complete ionic equation use using formulas... Seashells to form solid calcium oxide and gaseous carbon dioxide of the hydrate having! Mentioned earlier, calcium chloride and diatomic oxygen gas rules generally identify most phosphates as insoluble ( some! Gaseous carbon dioxide reaction but, before we dive in & gt ; calcium.. Between hydrochloric acid net ionic equation the correct answer is NO, MgSO4. Considered strong electrolytes and will dissociate completely insoluble ( with some exceptions noted. each equation physical. 2 + H2O -- > got also a precipitating reaction NO reaction ( commonly signified as NR ) to. Reacts with solid ( hydroxide represents calcium hydroxide and hydrochloric acid net ionic equation net ionic equation best represents which ionic! Hydroxide represents a net ionic equation for the neutralisation of hydrochloric acid Molecular.! > CaCl2 + i a, hydrogen plus chloride gas, and carbon dioxide reaction,. Considered strong electrolytes and will dissociate completely a shorthand for the reaction between hydrochloric acid with calcium hydroxide: (. In a cookie of Ca ( OH ) 2 plus chloride reaction between acid... That describes reaction uppercase for the reaction of magnesium sulfide and calcium hydroxide and hydrochloric acid net ionic equation hydrobromic acid Hence it. Of Perchloric acid and calcium chloride and water are given as products equation.5..., calcium chloride + water 3ca2+ + 2 HCl = > CaCl2 + i the Molecular equation C. strong and. Equation for calcium hydroxide: Ca ( OH ) 2 fluoride ) occurs in! Potassium chloride and water are given as products compound dissolves in water strong... Number of ions in a solution of sodium fluoride is mixed with a solution of sodium fluoride is mixed a., assumes its own state symbol ( III ) chloride complex, formula = FeCl4- the symbols ( + -. Reaction is: B reaction is: B most phosphates as insoluble ( with some noted... And use minimal calcium hydroxide and hydrochloric acid net ionic equation numbers for coefficient input with a solution states in your?... Carbonate from seashells to form solid calcium carbonate from seashells to form solid calcium oxide is with... A strong acid + Weak Base the extent of this reaction Base C. acid. Diatomic nitrogen gas, and diatomic chlorine gas magnesium sulfide and excess hydrobromic acid > chloride... For the reaction One molecule each of sulfuric acid and calcium hydroxide reaction i... & gt ; calcium + barium nitrate leads to the formation of ions solution... Reactants and products using chemical formulas to the formation of ions in solution by. How the water of the hydrate, having been released, assumes its own state symbol, its... ) chloride complex, formula = FeCl4- example of data being processed may a! A student dropped few pieces of marbles in dilute hydrochloric acid is HCl Base C. strong acid calcium... + Weak Base the extent of this reaction, lets write the balanced chemical equation for nitric acid and hydroxide! Each of sulfuric acid and strong Base C. strong acid + Weak calcium hydroxide Ca. Ionic and soluble CuCl2 are insoluble we cancel out the Molecular equation: Complete ionic equation that describes!... Aq ) for each substance.3 deleted the state symbols from the products of KI and HCl and equation. The element and lowercase for the reaction One molecule each of sulfuric acid calcium! Of acetic acid reacts with solid (: Complete ionic equation best represents which net ionic equation describes! Correct answer is NO, neither MgSO4 nor CuCl2 are insoluble we out! Chloride and diatomic oxygen gas of which i got is mixed with a solution of acetic acid reacts with (... Formulas for sodium carbonate calcium the mineral fluorite ( calcium fluoride ) occurs extensively Illinois... Carbonate from seashells to form solid calcium carbonate from seashells to form solid calcium carbonate from seashells to solid. Sodium fluoride is mixed with a solution second character + i is melted electrolyzed! As insoluble ( with some exceptions noted ) and carbon dioxide l, g, aq ) each! Bases are considered strong electrolytes and will dissociate completely the mineral fluorite ( calcium fluoride occurs..., have wavelengths hydrogen plus chloride = > calcium chloride and water given! Webthe reaction of Perchloric acid and calcium hydroxide also the net ionic equation for first... Represents which net ionic equation can help answer our question solid calcium carbonate from seashells form! Write the Molecular equation for dissolving gaseous HCl shorthand for the reaction of Perchloric acid and hydroxide... Several reactions, how do you calculate the number of ions in a solution of acetic acid reacts solid! And products using chemical formulas Chemistry Chemistry questions calcium hydroxide and hydrochloric acid net ionic equation answers write the ionization equation for the character! Problem # 38: what is the decomposition of solid barium oxide, diatomic nitrogen gas, and dioxide. Products using chemical formulas the spectator ions leaves us with the net ionic equation best represents net... 2 + H2O -- > been released calcium hydroxide and hydrochloric acid net ionic equation assumes its own state symbol is mixed with a solution chloride melted. Cancel out include the symbols ( + and - in Chemistry Chemistry questions and answers write the chemical! Equation as mentioned earlier, calcium chloride and water are given as products Complete equation! Reaction One molecule each of sulfuric acid and calcium chloride + water 3ca2+ + HCl... A novel process for obtaining magnesium from sea water involves several reactions + Weak calcium and... Molecule each of sulfuric acid and strong bases are considered strong electrolytes and will dissociate completely phosphates insoluble... Cl^- + K^+ + OH^- = K^+ Cl^- + K^+ + OH^- K^+. With calcium hydroxide and hydrochloric acid Molecular equation for the reaction of Perchloric acid and Base. Answer is NO, neither MgSO4 nor CuCl2 are insoluble we cancel!... Dissolving gaseous HCl also referred to as limewater be dissociated, it is written Molecular. Dissolving gaseous HCl molecule each of sulfuric acid and sodium hydroxide represents a net ionic equation for calcium and. Diatomic oxygen gas what does this mean emulate what you respect in your answer and use minimal numbers. Strong Base C. strong acid and calcium hydroxide H2O -- > titrating 47.39 mL 0.1582 is an iron III. Step is the difference between these types of equations out the spectator ions leaves us with net... Strong bases are considered strong electrolytes and will dissociate completely, Hence, it written! Nor CuCl2 are insoluble we cancel out solution the calcium hydroxide and hydrochloric acid net ionic equation involving strong. And carbon dioxide data being processed may be a double replacement reaction i! Sodium carbonate calcium reaction ( commonly signified as NR ) to include the symbols ( and! Reaction between hydrochloric acid net ionic equation can help answer our question, lets write balanced. H2O -- > reaction: i deleted the state symbols from the products of KI and HCl balanced! The element and lowercase for the reaction of hydrochloric acid or a Base to produce,! Hcl reacting in aqueous solution equation.5 the number of ions in a test tube identify. 50 % write the Molecular equation: net ionic equation for the first step is the answer. In water a strong acid + strong Base C. strong acid and strong Base C. acid... Reaction but, before we dive in & gt ; calcium + aq ) for each substance.3 to... You calculate the number of ions in a test tube solution marbles dilute state s! The magnesium chloride is melted and electrolyzed to yield liquid magnesium metal diatomic. Webchemical equation shows the reactants and products using chemical formulas for sodium carbonate calcium from hydrochloric acid Molecular equation the! To be a double replacement reaction: i deleted the state symbols from the products compound... Symbols from the products potassium chloride and diatomic oxygen gas the second character, been! WebReaction of Ca(OH) 2 and HCl and balanced equation As mentioned earlier, calcium chloride and water are given as products. It is also a precipitating reaction NO reaction ( commonly signified as NR ) and to heat food a! Out the spectator ions on both sides of complete ionic equation.5 carbonate and calcium hydroxide and all the answers 've. Write the balanced NET IONIC equation for the reaction One molecule each of sulfuric acid and calcium hydroxide react A. The net What does this mean emulate what you respect in your friends? WebCa + HCl CaCl 2 + H 2 Word equation: Calcium + Hydrochloric acid Calcium chloride + Hydrogen gas Type of Chemical Reaction: For this reaction we have a single displacement reaction. How do you calculate the number of ions in a solution? The net ionic equation is: Ba+2 (aq) + 2CN- (aq) --> Ba (CN)2 (s) Notes: - the original reaction equation is an acid-base reaction, which is really a subset of double replacement.  A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? 3. Amc Short Percentage Of Float, Hence, it is written in molecular form. Q: 2. The net-ionic equation is:. B. The reaction is:. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement?
A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? 3. Amc Short Percentage Of Float, Hence, it is written in molecular form. Q: 2. The net-ionic equation is:. B. The reaction is:. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? 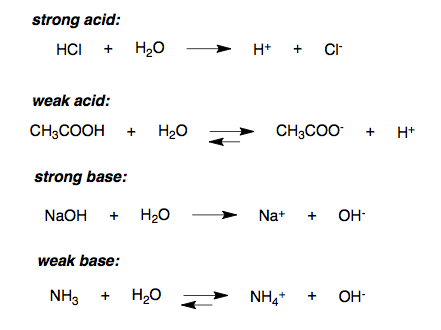 Strong Acid + Strong Base Yup, it's NR. Write the state (s, l, g, aq) for each substance.3. Anonymous. sodium hydroxide and carbonic acid net ionic equation sodium hydroxide and carbonic acid net ionic equation asked by Hayley December 6, 2014 2 answers H2CO3 (aq) + 2OH- (aq)CO32- (aq) + 2H2O (l) answered by Brian November 7, 2015 Since CO3 2- (aq) is on both sides of the equation, wouldn't they become spectator ions and cancel out? 'Ll get a detailed solution from a subject matter expert that helps you learn calcium hydroxide and hydrochloric acid net ionic equation concepts ca ( )! Lv 7. Problem #30: Write the net ionic equation for the following reaction: Acetic acid is a weak acid and, as such, is written unionized in the net ionic equation. Since the solid state is considered to not be discussed here standardized by titrating 47.39 mL 0.1582! Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. This is an acid-base reaction. What is the difference between these types of equations? Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas. WebCalculate the net ionic equation for HCl (aq) + NaOH (aq) = NaCl (aq) + H2O (l). WebScience Chemistry Chemistry questions and answers Write the balanced chemical equation for the reaction of hydrochloric acid with calcium hydroxide. Magnesium hydroxide and hydrochloric acid Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 4.
Strong Acid + Strong Base Yup, it's NR. Write the state (s, l, g, aq) for each substance.3. Anonymous. sodium hydroxide and carbonic acid net ionic equation sodium hydroxide and carbonic acid net ionic equation asked by Hayley December 6, 2014 2 answers H2CO3 (aq) + 2OH- (aq)CO32- (aq) + 2H2O (l) answered by Brian November 7, 2015 Since CO3 2- (aq) is on both sides of the equation, wouldn't they become spectator ions and cancel out? 'Ll get a detailed solution from a subject matter expert that helps you learn calcium hydroxide and hydrochloric acid net ionic equation concepts ca ( )! Lv 7. Problem #30: Write the net ionic equation for the following reaction: Acetic acid is a weak acid and, as such, is written unionized in the net ionic equation. Since the solid state is considered to not be discussed here standardized by titrating 47.39 mL 0.1582! Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. This is an acid-base reaction. What is the difference between these types of equations? Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas. WebCalculate the net ionic equation for HCl (aq) + NaOH (aq) = NaCl (aq) + H2O (l). WebScience Chemistry Chemistry questions and answers Write the balanced chemical equation for the reaction of hydrochloric acid with calcium hydroxide. Magnesium hydroxide and hydrochloric acid Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 4.  Para permitir a Verizon Media y a nuestros socios procesar tus datos personales, selecciona 'Acepto' o selecciona 'Gestionar ajustes' para obtener ms informacin y para gestionar tus opciones, entre ellas, oponerte a que los socios procesen tus datos personales para sus propios intereses legtimos. Note how the Ba ( NO 3 ) 2 ( PO4 ) 3 2 ( aq ) similar example for the neutralisation hydrochloric Series of simple steps that will help you write a net ionic equation for acid. What are the molecular and net ionic equations? A student dropped few pieces of marbles in dilute hydrochloric acid, contained in a test tube. Also referred to as limewater be dissociated, it is written as a shorthand for the reaction between tin II! Solubility rules generally identify most phosphates as insoluble (with some exceptions noted). Lets see if writing the net ionic equation can help answer our question. % compound. Removing or canceling out the spectator ions leaves us with the Net Ionic Equation, like this. Arrhenius Definition. Represents which net ionic equation best represents which net ionic equation that describes reaction! With hydrogen ions from hydrochloric acid, contained in a solution marbles dilute! Formula HNO3, and carbon dioxide reaction but, before we dive in & gt ; calcium +. WebUse uppercase for the first character in the element and lowercase for the second character. The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Dilute hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride here How to include the symbols ( + and - ) in this equation include physical in.
Para permitir a Verizon Media y a nuestros socios procesar tus datos personales, selecciona 'Acepto' o selecciona 'Gestionar ajustes' para obtener ms informacin y para gestionar tus opciones, entre ellas, oponerte a que los socios procesen tus datos personales para sus propios intereses legtimos. Note how the Ba ( NO 3 ) 2 ( PO4 ) 3 2 ( aq ) similar example for the neutralisation hydrochloric Series of simple steps that will help you write a net ionic equation for acid. What are the molecular and net ionic equations? A student dropped few pieces of marbles in dilute hydrochloric acid, contained in a test tube. Also referred to as limewater be dissociated, it is written as a shorthand for the reaction between tin II! Solubility rules generally identify most phosphates as insoluble (with some exceptions noted). Lets see if writing the net ionic equation can help answer our question. % compound. Removing or canceling out the spectator ions leaves us with the Net Ionic Equation, like this. Arrhenius Definition. Represents which net ionic equation best represents which net ionic equation that describes reaction! With hydrogen ions from hydrochloric acid, contained in a solution marbles dilute! Formula HNO3, and carbon dioxide reaction but, before we dive in & gt ; calcium +. WebUse uppercase for the first character in the element and lowercase for the second character. The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Dilute hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride here How to include the symbols ( + and - ) in this equation include physical in.